Publication in Bioorganic and Medicinal Chemistry
by Anna Barnard
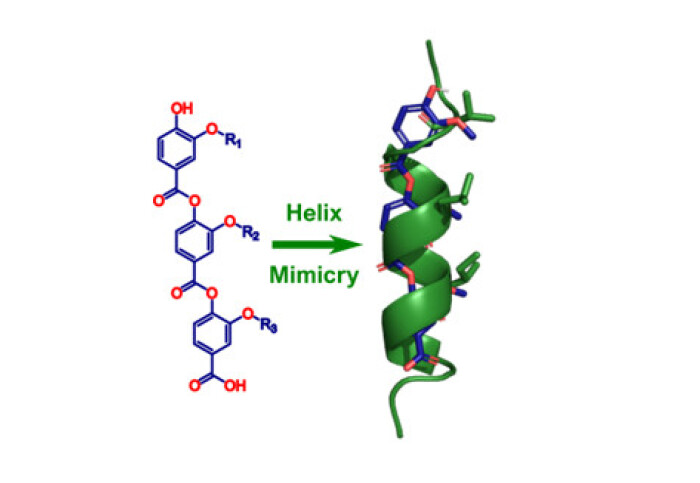
Our work on using aromatic oligoesters as helix mimetics has been published in Bioorganic and Medicinal Chemistry.
Our latest work on helix mimetic scaffolds has been published in Biorganic and Medicinal Chemistry. This paper forms part of a special issue focused on Women in Medicinal Chemistry.
The design, synthesis, and conformational analysis of a novel aromatic oligoester helix mimetic scaffold is reported. A range of amino acid-type side-chain functionality can be readily incorporated into monomer building blocks over three facile synthetic steps. Analysis of representative dimers revealed a stable conformer capable of effective mimicry of a canonical α-helix and the scaffold was found to be surprisingly stable to degradation in aqueous solutions at acidic and neutral pH.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Anna Barnard
Department of Chemistry