Infection of certain neurons with COVID could cause persistent symptoms
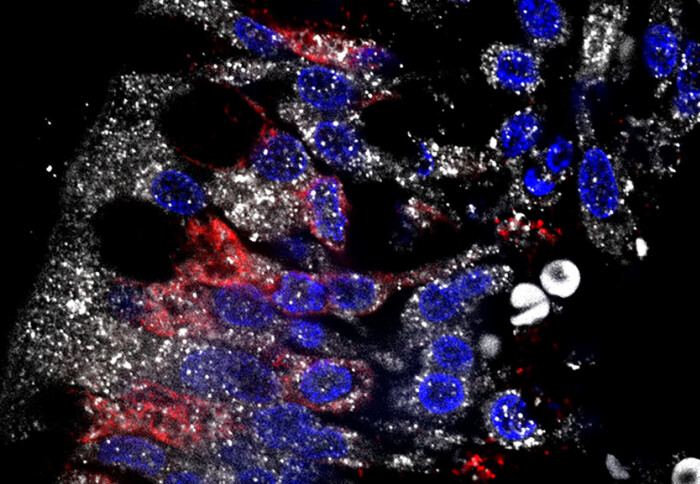
New research suggests that COVID-19 can lead to the death of neurons known for regulating sexual reproduction and cause persistent symptoms.
Researchers from Imperial College London, in collaboration with colleagues at Inserm, Lille University Hospital and Université de Lille, have been studying the impact of COVID-19 on a population of neurons in the brain known for regulating sexual reproduction via the hypothalamus (the neurons that express the GnRH hormone).
The findings, published in eBioMedicine, suggest that SARS-CoV-2 infection can lead to the death of these neurons and cause certain symptoms that persist over time.
Numerous scientific studies have documented the brain impacts of SARS-CoV-2 infection. One such effect is that a significant proportion of men have low testosterone levels that persist over time. Persistence beyond a period of four weeks is referred to as “long COVID”.
For many years, a research team from Inserm, Lille University Hospital and Université de Lille has been studying the role of certain neurons that express gonadotropin-releasing hormone (GnRH). From the hypothalamus, these neurons control all the processes associated with reproductive function: puberty, acquisition of secondary sexual characteristics, and fertility in adulthood.
These are the same scientists who had, for example, previously revealed that GnRH neuron dysfunction in an animal model of Down syndrome could affect the cognitive function impairment associated with this condition.
In this latest study, alarmed by the increasing number of reports showing that male COVID-19 patients also display persistent low testosterone levels even after recovery, the scientists wanted to test the hypothesis that SARS-CoV-2 infection may have harmful consequences on this population of neurons that regulate reproduction.
The Virus Penetrates GnRH Neurons and Alters Their Functions
Following hormone measurements (testosterone and luteinizing hormone) performed three months and then one year after infection in a small group of 47 men, the scientists observed that contact with the virus could alter the functions of GnRH neurons, leading to a fall in testosterone levels in certain patients some time after the infectious episode.
The scientists then wanted to verify whether the infection of the GnRH neurons and the subsequently observed hormone abnormalities could be associated with cognitive deficits. To do this, they listed the cognitive symptoms reported by the cohort patients, who underwent extensive testing three months and then one year after the infection.
The outcome was that the proportion of patients reporting memory or attention disorders, regardless of frequency or severity, and also concentration difficulties, tended to be slightly higher in the patients with abnormal hormone measurements, characterized by a decrease in testosterone levels.
Speaking about the findings, Professor Waljit Dhillo, and co-last author of this study and Head of the Division of Endocrinology and Metabolism in the Department of Metabolism, Digestion and Reproduction, said: “Although these were measurements made on a small sample of only male patients, these findings are very interesting and warrant further exploration in other larger-scale studies.”
To supplement their analyses, the researchers went on to study the brains of patients who died as a result of COVID-19. They identified the presence of the virus in the hypothalamus and the death of part of the GnRH neuron population.
“These findings may be worrying on several levels in terms of the role of these neurons in reproduction and their involvement in certain cognitive functions. They point to the necessity to optimize and generalize the medical follow-up of people with persistent symptoms following COVID-19 infection,” concludes Vincent Prévot, Inserm research director and co-last author of this study.
The study also encourages further research into the neurological impacts of long COVID.
This article was adapted from an Inserm new story.
Lead image © Vincent Prévot/Inserm
Long-COVID cognitive impairments and reproductive hormone deficits in men may stem from GnRH neuronal death. Sauve, Florent; Nampoothiri, Sreekala; Clarke, Sophie A; Fernandois, Daniela; Ferreira Coelho, Caio Fernando; Dewisme, Julie; Mills, Edouard G; Ternier, Gaetan; Cotellessa, Ludovica; Iglesias-Garcia, Cristina; Mueller-Fielitz, Helge; Lebouvier, Thibaud; Perbet, Romain; Florent, Vincent; Baroncini, Marc; Sharif, Ariane; Ereno-Orbea, June; Mercado-Gomez, Maria; Palazon, Asis; Mattot, Virginie; Pasquier, Florence; Catteau-Jonard, Sophie; Martinez-Chantar, Maria; Hrabovszky, Erik; Jourdain, Merce; Deplanque, Dominique; Morelli, Annamaria; Guarnieri, Giulia; Storme, Laurent; Robil, Cyril; Trottein, Francois; Nogueiras, Ruben; Schwaninger, Markus; Pigny, Pascal; Poissy, Julien; Chachlaki, Konstantina; Maurage, Claude-Alain; Giacobini, Paolo; Dhillo, Waljit; Rasika, S; Prevot, Vincent. eBioMedicine. 2023 DOI:https://doi.org/10.1016/j.ebiom.2023.104784
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Benjie Coleman
Department of Surgery & Cancer