S-acylation of p62 promotes autophagy and degradation of ubiquitinated proteins
In collaboration with researchers at Chongqing University, China, new findings in key S-acylation mechanisms have been uncovered.
A new paper in Molecular Cell, "S-acylation of p62 promotes p62 droplet recruitment into autophagosomes in mammalian autophagy" supported by Cancer Research UK, the Royal Society and the Chinese Natural Sciences Funding Council in collaboration with the team of Aimin Yang at Chongqing University, China, reports a key S-acylation mechanism underlying efficient autophagic degradation of p62 and ubiquitinated proteins, across a wide range of cellular models.
Autophagy is a natural cellular process that acts like a recycling system, cleaning up damaged or unwanted components within cells - the cell's way of "taking out the trash". p62, a well-known autophagy receptor, plays a vital role in recognizing and sequestering specific cellular "trash" into compartments called autophagosomes for degradation. It also aids in assembling and removing damaged proteins by forming p62-liquid droplets. However, the mechanism by which autophagosomes efficiently capture these p62 droplets has remained elusive.
This work reveals that p62 undergoes reversible S-acylation, a chemical modification, facilitated by the S-acyltransferase ZDHHC19. This modification enhances p62's affinity for microtubule-associated protein 1 light chain 3 (LC3)-positive membranes and promotes the localization of p62 droplets on autophagic membranes. This, in turn, results in the production of smaller LC3-positive p62 droplets and more efficient autophagic degradation of p62-cargo complexes.
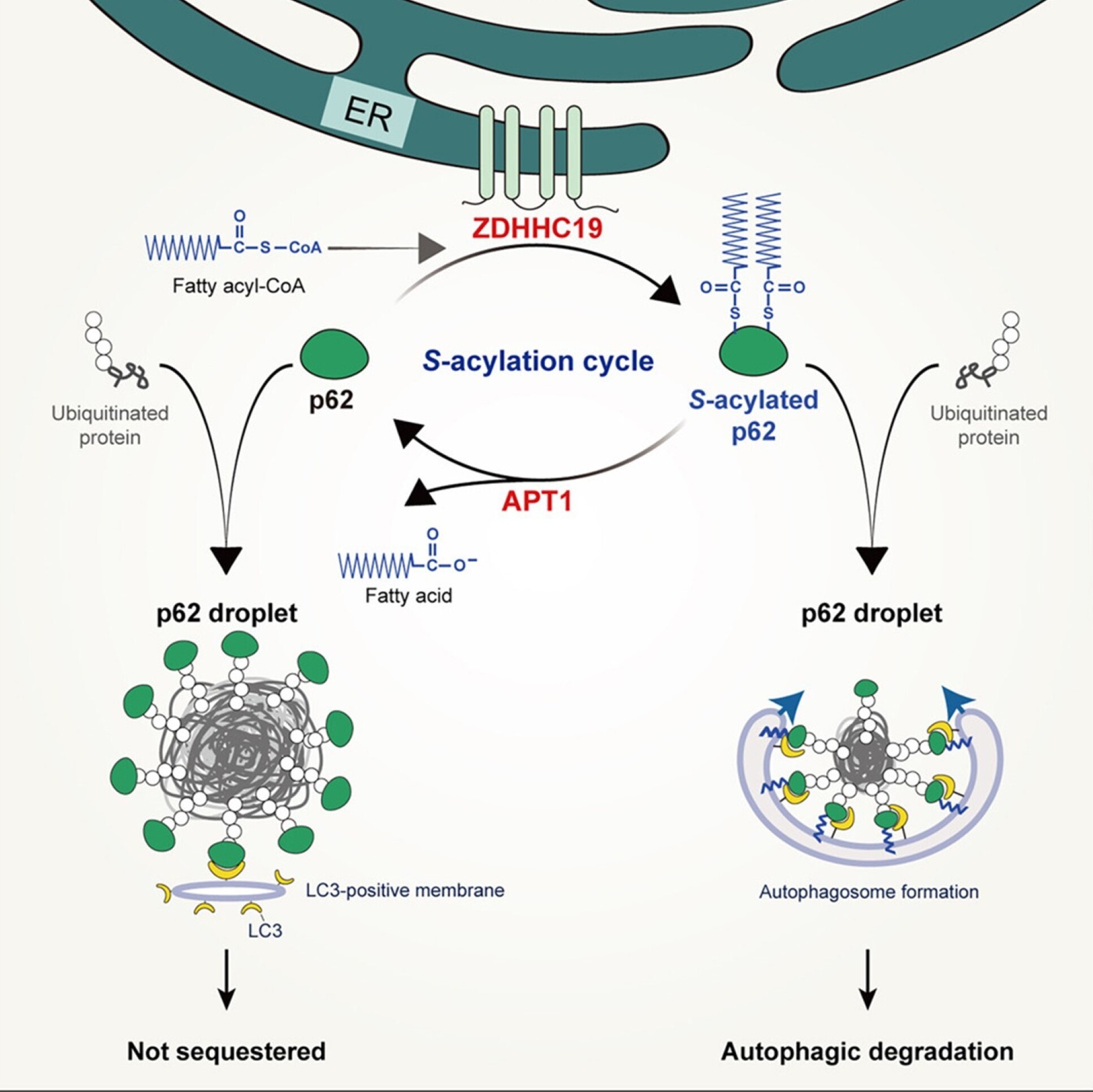
Schematic view of the S-acylation cycle resulting in p62 droplet recruitment.
We further demonstrated that increasing p62 acylation, either by upregulating ZDHHC19 or by genetically knocking out acyl protein thioesterase 1 (APT1), accelerates p62 degradation and the autophagic clearance of ubiquitinated proteins. In essence, the protein S-acylation-deacylation cycle plays a critical role in regulating p62 droplet recruitment to autophagic membranes and selective autophagic flux. This discovery holds significant implications for understanding and controlling the selective autophagic clearance of ubiquitinated proteins.
Cover Art: © Aimin Yang, Xue Huang, Zixin Shu, and Nanmu Zhang.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Dr Thomas J Burden
Department of Chemistry