Publication in ACS Chemical Biology
by Anna Barnard
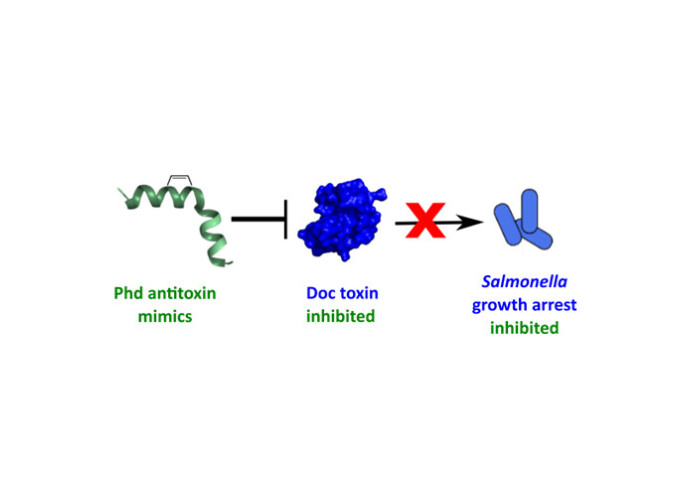
Our latest work on toxin-antitoxin interactions has been published in ACS Chemical Biology
Our research into the development of antitoxin mimetic peptides as toxin inhibitors has been published in ACS Chemical Biology.
Bacterial toxin inhibition is a promising approach to overcoming antibiotic failure. In Salmonella, knockout of the toxin Doc has been shown to significantly reduce the formation of antibiotic-tolerant persisters. Doc is a kinase that is inhibited in nontolerant cells by its cognate antitoxin, Phd. In this work, we have developed first-in-class stapled peptide antitoxin mimetics based on the Doc inhibitory sequence of Phd. After making a series of substitutions to improve bacterial uptake, we identified a lead stapled Phd peptide that is able to counteract Doc toxicity in Salmonella. This provides an exciting starting point for the further development of therapeutic peptides capable of reducing antibiotic persistence in pathogenic bacteria.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Anna Barnard
Department of Chemistry