Fighting neglected tropical diseases
by Lou Lee

To mark World Neglected Tropical Diseases Day, we explore how Imperial scientists are combatting these diseases that affect millions each year.
Neglected tropical diseases (NTDs) are a diverse group of bacterial, viral, and parasitic infectious diseases that impact over one billion people worldwide. They disproportionately affect those who are already vulnerable – whether through poverty, marginalisation, or geographic location – and cause significant suffering, disability, and even death.
World NTD Day is a vital opportunity to raise awareness of the devastating impact of NTDs on the world’s poorest populations. We look back at the past few years to see how Imperial’s researchers are supporting the control, treatment, and eradication of these diseases.
A new approach to detecting schistomiasis
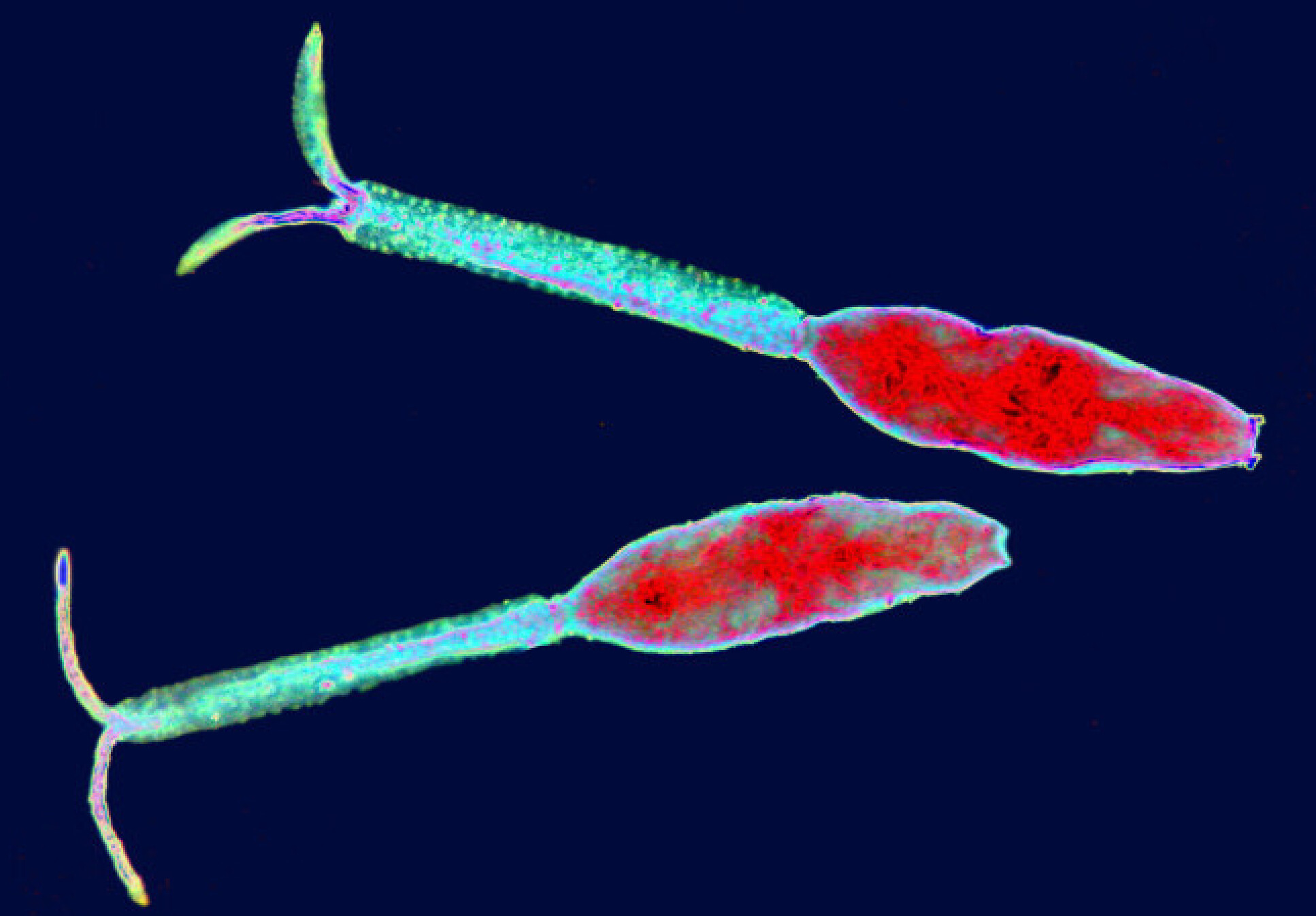
In 2022, an international team of scientists at Imperial, the National History Museum, Addis Ababa University in Ethiopia, and the National Institute for Medical Research (NIMR) in Tanzania, developed new DNA biosensors capable of rapidly detecting and differentiating between parasites that cause schistomiasis.
Schistomiasis is a debilitating NTD caused by parasitic worms, also known as schistosomes, that live in certain types of freshwater snails. The infection is endemic to regions within Africa, Asia and South America, and is particularly prevalent in poor communities without adequate sanitation.
The research findings suggest that the biosensors could be used as part of strategies to tackle the impact of schistomiasis at both a global and a local level.
Dr Alexander Webb, joint first author of the study and Research Associate at Imperial’s Department of Infectious Disease, said: “We are currently testing versions of our biosensor probes to detect drug resistance markers in schistosomes.”
Predicting visceral leishmaniasis relapse
In a study of patients co-infected with visceral leishmaniasis and HIV (VL/HIV) in Ethiopia, scientists looked to find out why some patients relapse frequently.
VL is caused by a parasite transmitted to humans by biting sandflies. While VL can be treated and cured, people with a compromised immune system – such as those with HIV – suffer frequent VL relapses.
Dr Pascale Kropf from Imperial’s Department of Infectious Disease, and colleagues, found that screening HIV patients’ blood could help to spot patients most at risk of relapsing from the parasitic disease, therefore improving disease management and patient care.
"This is extremely promising, as individuals who are most at risk of relapse … could be identified and treated further."
Preventing rabies with dog vaccination

Research led by academics at Imperial, the University of Glasglow and Ifkara Health Institute, found that rabies incidence in both humans and domestic dogs decreased during a period of sustained dog vaccination in southeast Tanzania.
Rabies is one of the world’s most feared diseases due to its high case fatality rate. Most human rabies cases result from bites by rabid animals, either from domestic dogs or, less commonly, wild animals including jackals. One exposed, immediate treatment is vital as rabies is invariably fatal once clinical signs develop.
The research team found that even in areas with a relatively high proportion of wildlife rabies cases, the domestic dog vaccination campaign still reduced the risk to humans. However, after mass dog vaccination ended in early 2017, rabies cases began to rise in some areas once again.
Sarah Hayes, one of the lead authors of the study from the School of Public Health, said: “It is critical that there is continued investment in domestic dog vaccination and this work suggests that the presence of rabies within wildlife populations should not be a barrier to implementing these programmes.”
Halting dengue transmission

A team of international scientists led by Imperial found that releasing mosquitoes infected with a type of bacteria that prevents them from transmitting dengue could cut cases of the disease by as much as 90%.
Dengue is a viral infection that infects over 100 million people each year. It is spread by mosquitoes and can sometimes lead to a life-threatening condition called haemorrhagic fever which is a leading cause of death and serious illness among children in some Asian and Latin American countries.
The Imperial team, based at the Jameel Institute within the School of Public Health, created the first ever global map of dengue transmission intensity. Using this map, the researchers predicted the global effectiveness of two interventions to combat dengue – vaccination and the release of ‘infected’ mosquitoes unable to transmit the virus between people.
Dr Lorenzo Cattarino, lead author of the research from the MRC Centre for Global Infectious Disease Analysis, said: “Our research can act as a tool to inform the World Health Organization, local governments and policy makers on the effectiveness of prevention strategies”.
Modelling the impact of climate change on dengue outbreaks

Modelling from Imperial and Tel Aviv University predicts that climate change could mean mosquitoes that can carry NTDs like dengue and chikungunya become established in southern Europe within 10 years.
The work suggests that rising temperatures and changing rainfall patterns are increasing the number of areas the disease-carrying mosquitoes can live in, potentially spreading diseases to new places including parts of China, North America and European countries such as Spain and Greece.
Dr Kris Murray, from the MRC Centre for Global Infectious Disease Analysis in the School of Public health and the Grantham Institute – Climate Change and Environment at Imperial, said: “This work helps reveal the potential long-term costs of failing to curb greenhouse gas emissions right now.”
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Lou Lee
Faculty of Medicine Centre