First small molecule activity-based probes for USP30 discovered
Tate group researchers have produced the first small molecule activity-based probes for USP30.
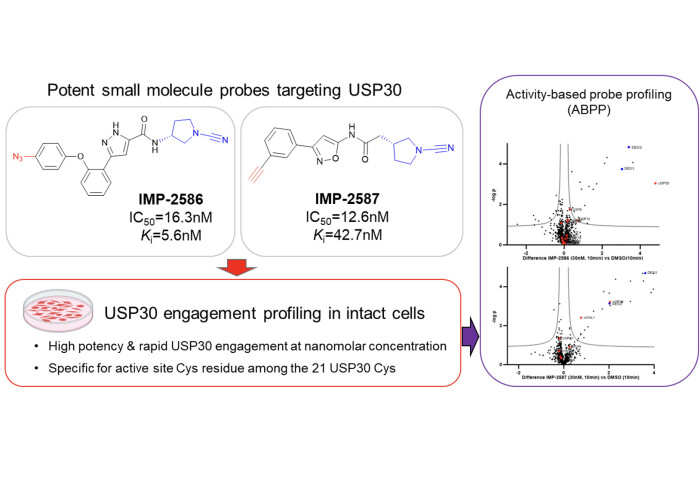
Past and present Tate group researchers Milon Mondal, Fangyuan Cao and Daniel Conole have published a study in RSC Chemical Biology reporting development of the first selective small molecule activity-based probes (ABPs) for USP30, enabling profiling of USP30 deubiquitinase (DUB) activity in intact cells for the first time.
USP30, identified as a promising target for drug development, is a DUB – an enzyme which regulates turnover and function of substrate proteins by removing the post-translational modification ubiquitin, frequently resulting in stabilisation of the substrate. USP30 plays crucial roles in mitophagy, pexophagy, apoptosis, and tumorigenesis, and recent advancements have seen a USP30 inhibitor progressing into clinical trials as a potential treatment for mitochondrial dysfunction. Our paper offers robust molecular tools to expedite drug development and deepen our understanding of USP30 activity in a cellular context.
The ABPs IMP-2586 and IMP-2587, equipped with a cyanopyrolidine warhead, exhibit remarkably high potency and selectivity for USP30 over the 80+ DUBs in the proteome (the “DUBome”). Through comprehensive biochemical evaluation, target engagement studies, and proteomics analysis in cells, we demonstrate that these probes effectively capture USP30 activity at low nanomolar probe concentrations. Additionally, further investigations confirmed selective engagement of the active site cysteine of USP30 in cells. Together, these probes offer promising prospects for the future development of therapeutic interventions targeting USP30.
This research was funded by the European Commission, the Laboratory for Synthetic Chemistry and Chemical Biology under the Health@InnoHK Program of The Government of Hong Kong Special Administrative Region of the People’s Republic of China and the Cancer Research UK Imperial Centre at Imperial College London.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Dr Thomas J Burden
Department of Chemistry