Discovery of first generation of covalent VHL-recruiting PROTACs
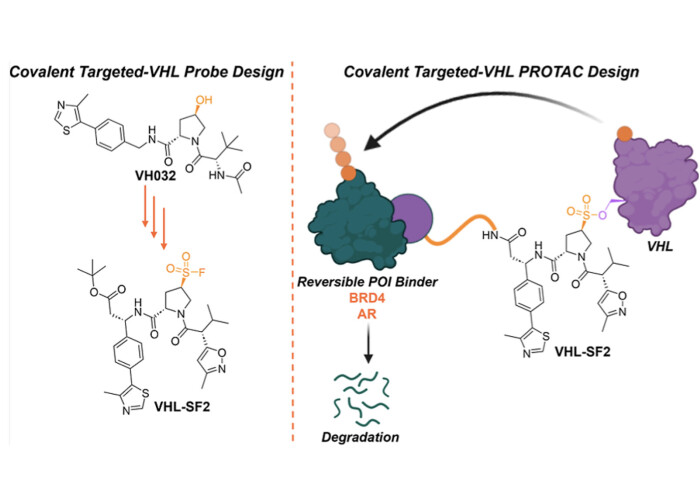
We have reported the first generation of covalent VHL-recruiting PROTACs, now out in J. Med. Chem.
In a challenging piece of structure-guided optimisation led by Rishi Shah during his UK Research and Innovation-funded secondment from GSK in our lab, this study published in J. Med. Chem. introduces a significant advance in the development of covalent E3 ligase binders through structure-guided design, rather than random screening methods. Unlike many covalent ligands which target cysteine residues, our VHL ligand interacts covalently with the Ser110 residue of VHL through a sulfonyl fluoride group, marking the first instance of a VHL ligand devoid of a hydroxyproline motif. This innovation opens new avenues to enhance the pharmacokinetic and pharmacodynamic properties of VHL-targeted PROTACs and expand the substrate range of E3 ligase-covalent PROTACs to the wide range of degradation targets of VHL PROTACs. Covalent VHL PROTACs also offer advantages such as sustained degradation at low stoichiometry, decoupling degradation efficiency from VHL occupancy.
Our sulfonyl fluoride-based PROTACs demonstrate the ability to induce targeted protein degradation (TPD) of BRD4 and AR in a proteasome- and ubiquitin ligase-dependent manner. While functional degradation is observed in these first-generation, unoptimized prototypes, the degradation efficiency falls short of highly optimized noncovalent PROTACs; future investigations will be necessary to optimise these PROTACs and understand the effects of adduct population on the natural substrates of VHL.
Congratulations to Rishi (who is now a collaborator at Ubiquigent), the protein degradation team at GSK, Elena De Vita and Daniel Conole from our lab, and Xinyue Zhang for her first paper in our lab.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Dr Thomas J Burden
Department of Chemistry