Trial into ventilation strategies receives £2.1 million funding from NIHR
A new Imperial led trial will explore better treatment strategies for patients with acute respiratory distress syndrome.
The trial, "Rest Or Moderate Mechanical Ventilation During ECMO Support" (ROMEO), has received £2.1 million in funding from the National Institute for Health Research (NIHR), to examine improving treatment for acute respiratory distress syndrome (ARDS) patients requiring extracorporeal membrane oxygenation (ECMO).
"The research holds the promise of significantly improving outcomes for some of the most critically ill patients.” Dr Brijesh Patel
Each year, approximately 30,000 adults in the UK are placed on ventilators due to ARDS, a condition causing life-threatening respiratory failure. While ventilators are essential for keeping these patients alive, they can also cause ventilator-induced lung injury (VILI).
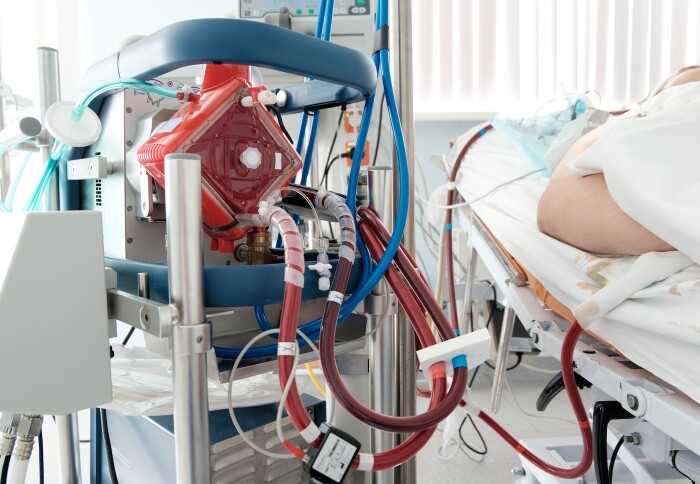
In severe cases where patients continue to have dangerously low oxygen levels, an additional artificial lung machine, known as an extracorporeal membrane oxygenation (ECMO) machine, is required. The ECMO machine oxygenates the blood and removes carbon dioxide, allowing the ventilator settings to be adjusted to less harmful levels.
The multi-centre randomised controlled trial ROMEO trial, led by Dr Brijesh Patel, a Clinical Senior Lecturer in Cardiothoracic Critical Care in the Department of Surgery and Cancer, seeks to investigate if reducing the number of breaths delivered by a ventilator to near zero during ECMO support can promote lung recovery.
Current research suggests that gentler ventilation, with a very low respiratory rate of two breaths per minute, could potentially minimize VILI and improve patient outcomes compared to the standard ventilation rate of 10-20 breaths per minute.
Speaking about the trial, Dr Patel said: “The primary goal is to determine if the reduced breathing rate leads to a shorter duration of ECMO support, signifying faster lung recovery. We will also assess complications associated with prolonged ECMO use, such as bleeding, blood clots, and strokes.
“Our hope is that the trial findings can lead to better management strategies for ARDS patients on ECMO support worldwide. The research holds the promise of significantly improving outcomes for some of the most critically ill patients.”
The ROMEO trial also includes plans for long-term follow-up of participants to evaluate mortality, duration of ventilator use, ECMO complications, and time spent in the ICU or hospital. This extended observation will help to comprehensively understand the effects of reduced ventilation rates on patient recovery and overall health.
The ROMEO trial is a collaboration between Imperial College London, Royal Brompton and St Thomas’ Hospitals (part of Guy's and St Thomas' NHS Foundation Trust), King's College Hospital NHS Foundation Trust, Johns Hopkins University and Queen's University Belfast, and is the first major trial involving the NHS's highly specialist commissioned national UK ECMO Network for severe acute respiratory failure.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Benjie Coleman
Department of Surgery & Cancer