Mar 2024 - Article Published in Org. Lett.

Org. Lett.
Alex Boddy and Adi Sahay's development of an enantioselective phase-transfer approach to spiroxindole azetidines is published in Org. Lett.
A.J. Boddy, A.K. Sahay, E.L. Rivers, A.J.P. White, A.C. Spivey and J.A. Bull, ‘Enantioselective Phase Transfer Catalyzed Synthesis of Spirocyclic Azetidine Oxindoles’, Org. Lett, 2024, 26, 2079-2084. DOI: https://doi.org/10.1021/acs.orglett.4c00358
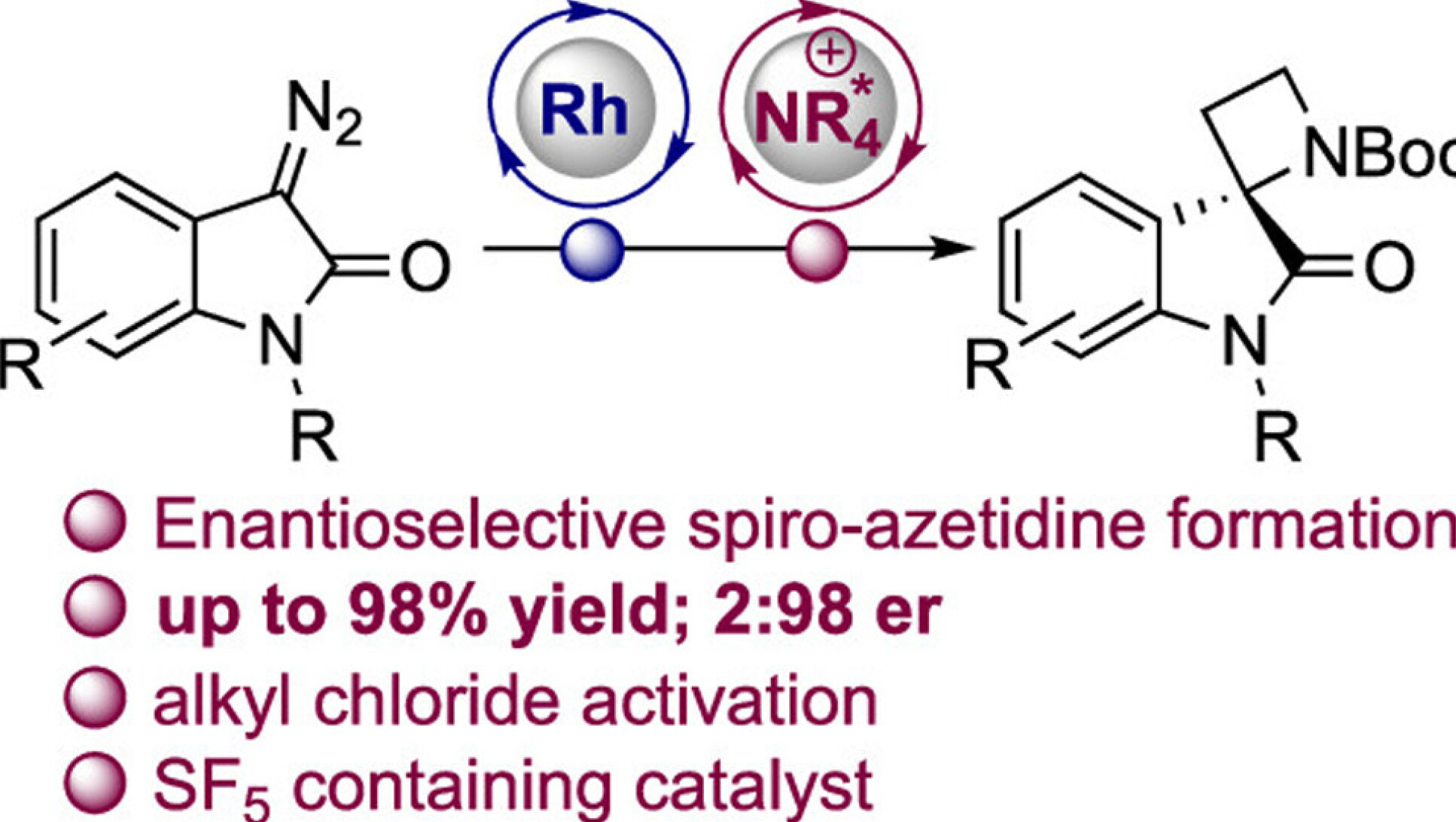
Spiro-3,2′-azetidine oxindoles combine two independently important pharmacophores in an understudied spirocyclic motif that is attractive for medicinal chemistry. Here, the enantioselective synthesis of these structures is achieved in up to 2:98 er through intramolecular C–C bond formation, involving activation of the substrate with a novel SF5-containing chiral cation phase-transfer (PT) catalyst. The products are readily elaborated/deprotected to afford medicinally relevant enantioenriched compounds. Control experiments suggest an interfacial PT mechanism, whereby catalytic asymmetric induction is achieved through the activation of the chloride leaving group.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Professor Alan C Spivey
Department of Chemistry