Key info
Date:
18-21 June 2020
Activity:
Online public involvement survey for parents/carers of children aged 5-17 and young people aged 16-17 to share their views on the acceptability of antibody testing on children.
Insight Report authors:
Philippa Pristerà, Emily Cooper
Overview
The REACT study, which stands for REal-time Assessment of Community Transmission, is a major programme of home testing for COVID-19 to track the progress of the infection across England. It was commissioned by the Department of Health and Social Care and is being carried out by Imperial College London in partnership with Imperial College Healthcare NHS Trust and IpsosMORI. The Patient Experience Research Centre (PERC) are supporting the antibody testing usability studies as part of this programme of work and leading the public involvement activity that is informing its design and management.
An important aim of this programme is to estimate how many people in England have already been infected with the virus that causes COVID-19. This involves getting people to do a finger-prick antibody test at home to check for antibodies in their blood. The presence of antibodies (i.e. a positive test result) would suggest someone had already had COVID-19 in the past. However, our studies to date have only explored how easy it is for adults (aged 18+) to use these finger-prick antibody tests correctly at home. Few studies have explored how acceptable or easy it is to perform these finger-prick tests on children. Enabling these tests to be performed on children would give researchers a much greater understanding of how the virus has spread through the population. We feel further research is needed to understand the usability of performing this finger-prick antibody test on children, therefore we turned to parents, carers and young people to get their thoughts and guide any plans going forwards.
Between 18-21 June 2020, we carried out an online public involvement survey to capture the views of parents, carers and young people across England and received 4,290 responses. Questions aimed to gather the reasons why people would or would not want to test their child (or themselves for young people), whether they would do the test for research purposes only, their preferences on the testing approach (including where the test should be carried out and by who) and how the instructions and material could be made more suitable.
Key Insights
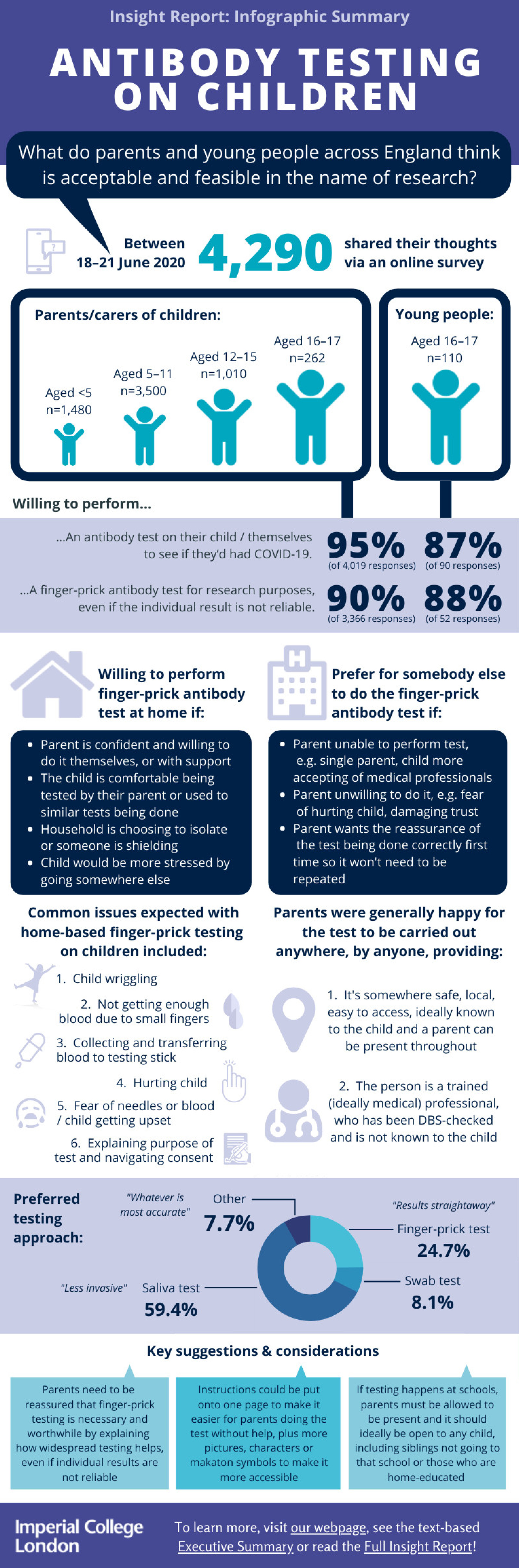
Of the 4,290 responses received, 4,180 were from parents/carers (of children aged 5-17) and 110 were young people (aged 16-17). Of the parents/carers, 23.7% had children aged under 5 (n=1,480), 56.0% had children aged 5-11 years old (n=3,500), 16.2% had children aged 12-15 years old (n=1,010) and 4.2% had children aged 16-17 years old (n=262).
- There is high willingness among parents/carers to perform finger-prick antibody testing on children, even if just for research purposes.
➢ 95% said they would want to perform an antibody test on their child to see if they'd had COVID-19
➢ 81-96% said they would be willing to perform a finger-prick antibody test on their child, including those aged under 5 (81%; willingness increasing with age group)
➢ 90% were still willing to perform a finger-prick test on their child as part of research (i.e. to help researchers monitor the spread of the virus even if it didn’t provide an accurate individual result)
➢ The consensus was that children of any age should be tested, starting with older children. However, this likely linked to parent’s eagerness to know if their child had already had COVID-19 and/or had maintained antibodies with the view that it might influences their behavioural choices. Clarity is therefore needed about the purpose and benefits of widespread antibody testing, and what it cannot tell us
➢ Several parents commented that they would like enough kits to test the whole family/household, both so they knew what the finger-prick felt like before doing it on their child and for some level of perceived validation and reassurance about the result, despite being informed it may not be reliable at an individual level - Young people also showed high willingness to be tested to see if they've had COVID-19, although to a slightly lesser extent than parents/carers.
➢ 87% said they would test themselves to see if they'd had COVID-19
➢ 74% said they would be willing to perform a finger-prick test on themselves
➢ 88% were still willing to perform the finger-prick on themselves as part of research - Overall preference was for the test to be performed at home by the parent or young person.
➢ Parent’s ability to test their child was highly linked to the child’s character and/or the parent’s confidence to perform the test. However, under 3s were generally perceived to be the most difficult to test due to them not staying still and being hardest to communicate with
➢ Parents were more willing to perform the test themselves if they were a healthcare professional or their child was used to doing finger-prick tests/blood glucose monitoring and other medical procedures. They were less willing to perform the test themselves if their child was very anxious or aged under 18 months
➢ 59.2% and 36.6% of parents/carers said they would ask for help or support from someone else in their household if performing the test on a child under 5 and aged 5-11, respectively. The primary reason was to have someone to distract and comfort the child while the other read the instructions and performed the test. 54.3% of young people said they would seek help from someone in the household, most commonly the mum
➢ Some parents/carers who had children with sensory issues said the home environment would be much better for their child, while others felt a medical professional, and setting, would be better
➢ Home-based testing was particularly preferred by parents who were trying to isolate as much as possible or were themselves shielding or had someone in the household who was shielding due to a clinical vulnerability to COVID-19. However, comments suggested they would be prepared to go elsewhere if they had to, providing the location was safe - If home-based testing wasn’t possible or people wanted an alternative, the consensus was that it should be carried out by a trained professional, ideally somewhere local.
➢ Preference was for it to be a medical professional (e.g. nurse, healthcare assistant, health visitor, pharmacist), but most seemed happy to accept “anyone” providing the person was:
- Adequately trained to do the test correctly and safely
- DBS-checked
- Used to dealing with children
- Ideally not known to the child (unless they were the child’s routine healthcare staff)
➢ The preferred location would local and/or known to the child or parent (e.g. school, community centre, village hall, pharmacy, hospital clinic as part of child’s routine care). However, once again generally “anywhere” was considered acceptable providing:
- The parent could also be present
- It was safe and well-managed (i.e. social distancing possible, strict appointment window, no queuing, minimal contact with others)
- It was local, convenient and easy to get to
- It was child-friendly and preferably somewhere that they were already visiting
➢ Primary reasons for wanting the test done by someone else included: parent feeling uncomfortable or unable to do it themselves; wanting the certainty that it was done correctly and wouldn’t need to be repeated; not wanting to damage the trust or relationship with their child; expectation that their child would behave better; feeling that it would be quicker and less stressful for the child
- The most common anticipated challenges of performing the test on children included:
➢ Child moving or wriggling (dependent on child but more common concern for under 5s)
➢ Not getting enough blood (more common concern with younger children due to small finger)
➢ Trouble transferring the blood to the testing stick due to child moving or getting upset
➢ Fear of hurting the child or causing pain
➢ Child’s fear of needles or seeing blood (more common among older children and young people)
➢ Difficulties explaining why it was being done and why it was important or necessary, especially with children who have sensory issues
➢ A key concern specific to young people was performing the test correctly and one-handed - Several parents raised the importance of, and sometimes difficulties around, gaining informed consent for the different age groups. Comments shared about their decision making included:
➢ Children under 5: ability to do the test would depend on their child’s nature and their ability to communicate with them. Parents would not want to do the test if they were resisting
➢ Aged 5-11: Parents of children in this age talked more about their ability to explain the test to their child to encourage them to take an active part in the process
➢ Aged 12-15: Fear of needles was a primary reason why parents thought their 12 to 15-year-old would not do the test. There were also suggestions around the difficulty of persuading a teenager to do things. It was felt it would be their decision, not the parent’s
➢ Aged 16-17: Parents of this age group generally felt their child would be able to decide and do the test themselves
- Overall, the materials were seen to be appropriate for all age groups although many felt it was more likely that the parent would use them rather than the child directly, especially under 5s.
➢ Suggested improvements to make it easier for parents to use the instructions with their child included providing the tools to:
- Explain the test: why the test is needed and how it helps; less words, more pictures; brighter images; storyboard style including a cartoon hero or well-recognised character; Makaton symbols; more child-friendly language and images
- Carry out the test: distraction for the child e.g. a video, something online, second member of household; simplify instructions onto one larger page to make it easier for parents performing the test by themselves
- Reward their child: e.g. a sticker, ideally as sparkly as possible or including cartoon hero or known character. Some suggested including a child-friendly (cartoon) plaster too - Overall preferences and comments on the type of test:
➢ 59% chose “saliva test (spit into tube)” as their preferred test approach, followed by “finger-prick test (as shown)” (25%); many liked being able to get the test result themselves straightaway. Further comments suggested preference was generally for whichever test is more accurate.
➢ However, “swab test (nose and throat)” was only voted for by 8% of respondents suggesting people would want the best compromise between accuracy and experience/usability. Some said they would be happy with 85% accuracy, others said it would need to be >95%. It was also noted that spitting is generally discouraged in children therefore they were not keen to now encourage that for this test in case children mimicked it later. In addition, some felt it would be almost impossible to get a child aged under 2 or 3 to spit at all - Key considerations and comments raised at the end of the survey included:
➢ Clarifying the purpose of the study, which included explaining how it’s possible to understand the spread when the result is not accurate at an individual level and reassuring parents that it is necessary and worthwhile
➢ Ensuring transparency about what data would be collected, how it would be stored and whether it would be anonymous. And how people will be able to access the population-level data
➢ Queries about the accuracy and meaning of the test result, including possible long-term consequences of a positive result
➢ Reiterating the support and urgency for this study
➢ Further suggestions on how to improve the instructions for children and queries about the test itself, including health and safety
What next
- After the survey closed, we shared a high-level summary of the responses with MHRA (the Government’s Medicines and Healthcare products Regulatory Agency) and the Department of Health and Social Care (DHSC). This aimed to demonstrate the overall support for antibody testing on children, even for research purposes, and overall preferences that should be considered if testing goes ahead
- The finalised Insight Report and supporting Infographic and Executive summaries have now also been shared with those at DHSC, Ipsos MORI and Imperial College London who are involved in ongoing planning and management of the antibody testing programme
- The Department of Health and Social Care and Imperial College London are continuing to plan the next phases of the REACT-2 antibody testing programme, which includes ongoing conversations about extending testing to people in care homes and to children. What we've learnt from this involvement activity will help to guide this planning, as well other areas of research into antibody testing more generally
- In the meantime, antibody testing of the general adult population will continue in waves throughout the year as part of REACT-2 Study 5. Adults in England will be selected randomly from the NHS database and sent an invitation letter to take part
- Further information and updates on the REACT study will be available on Imperial’s REACT study webpage
- And where necessary, we will post updates online about our further community involvement and via Twitter @Imperial_PERC
Further reading
- For related findings, read our Antibody Testing Insight Report from 1 May 2020, which captured people's early views on the REACT study and antibody testing on adults
- To learn more about the REACT testing programme, visit the study website
Read our blog
All postsContact us
PERC Director and Co-Founder
Prof. Helen Ward
h.ward@imperial.ac.uk
For enquiries about public involvement in research and research more generally, please email:
publicinvolvement@imperial.ac.uk