For Imperial College London, ethics reviews mean ensuring that a research project is conducted in such a way that any potential adverse impact is in proportion to its expected benefit. Adverse impacts are those that may affect the environment and/or the health, well-being, safety, threats to privacy and in rare cases liberty of individuals and/or the reputation of the College.
We must ensure that a research project is conducted in such a way that any adverse impact is in proportion to its expected benefit.
Adverse impacts may consist of acknowledged potential direct risks (such as rare or adverse reaction to researcher intervention), unintended consequences (such as stigmatisation of an individual through involvement in research).
The expected benefits must not only be desirable but also in sufficient degree likely to occur. The benefits of the research are a material consideration in deciding whether a proposal is ethical as presented since they must be in satisfactory proportion to the potential adverse impacts.
Research ethics forms an integral part of proper scientific conduct, and we expect all staff and students to adhere to.
Ethics overview
- Do I need to apply?
- If yes, then why must I apply?
- When don't I need to apply?
- Other study types
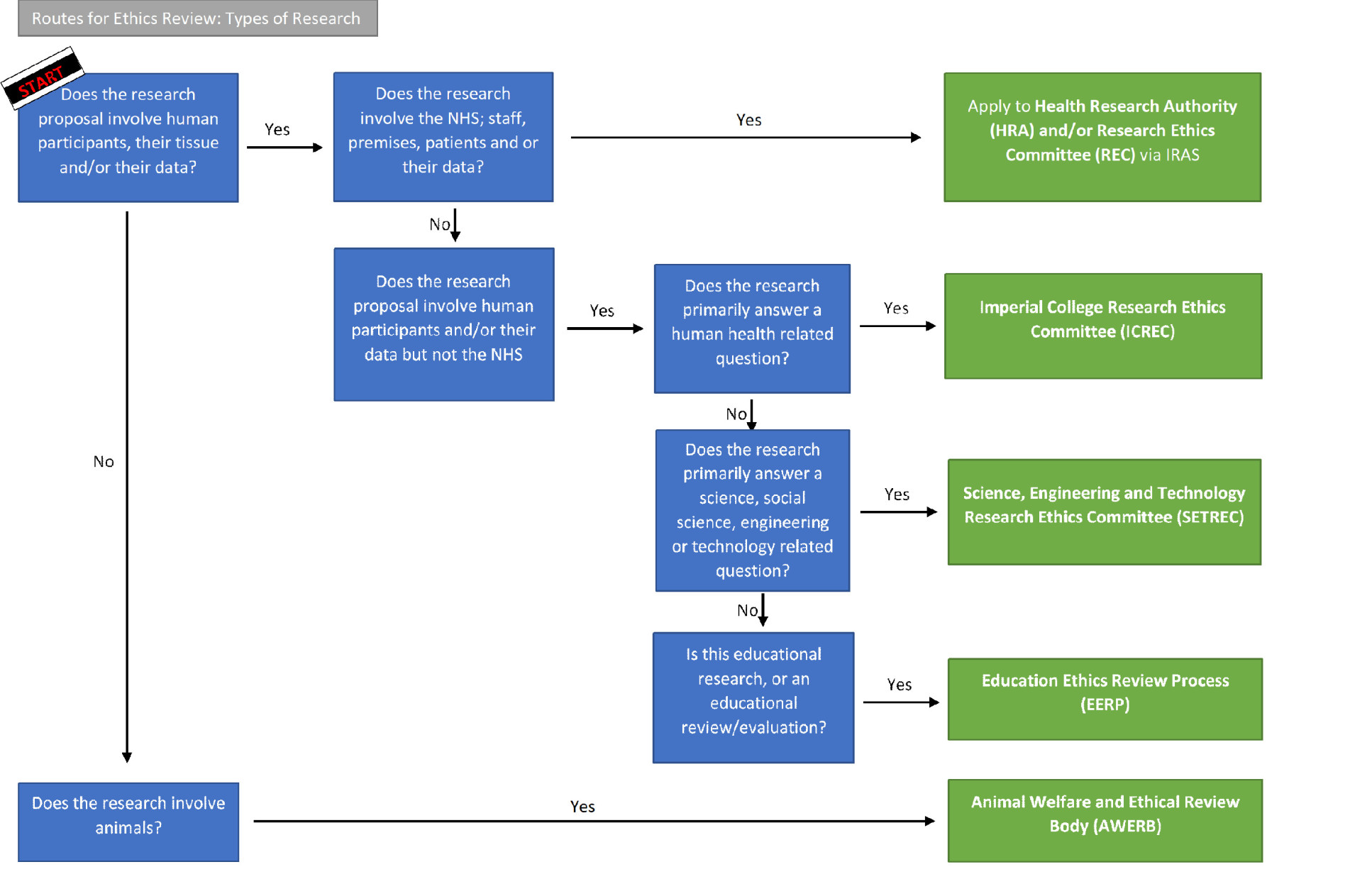
- Routes to ethics review
 Ethics approval is needed for any research that involves human participants; their tissue and /or data to ensure that the dignity, rights, safety and well-being of all participants are the primary consideration of the research project.
Ethics approval is needed for any research that involves human participants; their tissue and /or data to ensure that the dignity, rights, safety and well-being of all participants are the primary consideration of the research project.
Being ‘ethical’ means acting in accordance with a set of core values and principles, in particular, integrity, compliance with the law, respect for human rights and avoiding unnecessary risk to people’s safety and well-being. Imperial College London seeks to ensure that any potential ethical risks arising from research are limited strictly in proportion to the importance of the intended benefits.
A researcher must, therefore, consider the ethical implications of any work that:
- has the potential to damage the mental or physical health of human participants, (e.g. volunteers, College staff and students, or patients,) or others who may be affected
- has the potential to jeopardise the safety and liberty of people affected by the research (e.g. volunteers working in sensitive situations or abroad)
- has the potential to compromise the privacy of individuals whose data is involved in the work. Personal data must remain unknown to all but the research team (unless the participant agrees otherwise or in cases where there is an overriding public interest, or where participants wish their voices to be heard and identified).
- involves methods (e.g. genetic research, interviews, questionnaires, randomised control trial) or subject matter (e.g. recreational and controlled drugs, human impact on the environment) that are sensitive and therefore need to be managed consistently with the College's high public reputation
- carries a risk of an actual or perceived conflict of interest on the part of researchers and/or the College. This also includes considering the legitimate interests of other stakeholders.
- this includes secondary data from researchers including anonymised data, pseudonymised data and identifiable data
- Has a potential dual purpose in addition to the intended purpose (in these cases refer to the Export control website)
 Full approval is a mandatory requirement of the College for all research it sponsors and must be sought before the start of the research project. Conducting research without such approval constitutes misconduct, and the College takes no responsibility, financial or otherwise for this.
Full approval is a mandatory requirement of the College for all research it sponsors and must be sought before the start of the research project. Conducting research without such approval constitutes misconduct, and the College takes no responsibility, financial or otherwise for this.
- The RGI team and the committees (ICREC and SETREC) and the Educational Ethics Review Process (EERP) cannot grant approval retrospectively. Only data collected after ethics approval has been given can be used. Any data collected before this must not be used.
- Analysis of secondary data requires ethics approval; whether the data is sensitive or anonymised (this does not include publicly available data).
- Unless you can demonstrate that your project is not research you should apply for ethical approval.
The Committees and the RGI Team do not review the following: |
|
|
|
|
ICREC and SETREC do not give ethics reviews for research in which the College is not the sponsor and is a collaborator/College researcher is a Co-Investigator, unless interventionist procedures will be taking place by the Co-Investigator at the College (where the College is a site). However, the Co-Investigator must ensure that the Principal Investigator gains ethical approval from his/her own institution before the research begins.
*Pedagogic research is research where the theoretical framing of the research question concerns teaching and learning and the research question itself investigates an aspect of teaching or learning. The EERP would not consider a research proposal as pedagogic simply because it was performed by a student for academic credit or as part of an Imperial College academic programme or module.
** High risks educational projects will initially be reviewed by EERP and then sent to Science Engineering and Technology Research Ethics Committee (SETREC) for review.
 If your study falls under the following criteria then you do not need to apply:
If your study falls under the following criteria then you do not need to apply:
Service evaluation
Service evaluation is undertaken to benefit those who use a particular service and is designed and conducted solely to define or judge current service. The participants will normally be those who use the service or deliver it. It involves an intervention where there is no change to the standard service being delivered (e.g. no randomisation of service users into different groups).
Audit
Audit is defined as assessing the level of service being provided against a set of pre-determined standards. This generally involves analysing existing data with results usually being used/distributed locally in order to effect change to improve/change the level of service currently being provided.
Surveillance
Surveillance is designed to manage outbreak and help the public by identifying and understanding the risks associated with the outbreak. It is designed to answer the following question “What is the cause of this outbreak?”.
More guidance can be found at: decision table for research, service evaluation, audit and surveillance[Word].