Research
Photovoltaics
-001crop--tojpeg_1519204149876_x1.jpg)
The Durrant group solar cell team has a primary research interest in the development and understanding of low cost, printed photovoltaic technologies for harnessing solar energy to produce electricity. It has previously studied on dye-sensitised solar cells; more recently the team has focused on solar cells fabricated from organic polymers and small molecules (OPV) and, since 2014, perovskite solar cells. The image highlihgts the brilliant range of colours OPV can be, including some exciting non-fullerene acceptor (NFA) materials that the group is currently working on. These technologies have the potential to be efficient, light weight and flexible whilst being manufactured at low cost on a large area through solution processing.
The group undertakes fundamental scientific studies of new materials and device concepts, aiming to elucidate design principles which enable technological development. Our work addresses both material thin films and complete devices. One of the main branches of research within the Durrant group is based around using transient laser spectroscopies to undertake photochemical and photophysical studies of light driven electron and energy transfer reactions on relevant timescales. These experiments allow the study of the fundamental charge generation and recombination processes that occur within a solar cell. These spectroscopic studies are supplemented with a range of opto-electronic experimental techniques, these measure the electronic response of a complete solar cell to various illuminations. These techniques offer a broad spectrum of analytic tools that have proved vital in analysing recombination loss processes that define the power conversion efficiencies of organic solar cells. These studies are corrlated with studies of materials structure on the nanometre length scale and therefore the nano-morphology and the use of nanostructured materials is a key component of our research.
In addition to experiments aimed at improving the power conversion efficiencies of organic photovoltaic devices, the group studies the degradation processes that reduce the performance of solar cells during their operating lifetime. An understanding of this process is essential if these devices are to be manufactured, and the group utilises equipment for the aging of cells and analysis of these processes.
The group's solar cell activities are undertaken as part of Imperial's Centre for Processable Electronics, enabling extensive collaborations across different departments, including with groups working on material synthesis, processing, nanomorphology, device physics and modelling. These activities are also aligned with and complimented by the scale up activities of the SPECIFIC IKC in Swansea, particularly for perovskite solar cells.
Solar-driven fuel synthesis
The growing energy demand from our society requires the development of a sustainable, environmentally clean and secure energy source and storage system. In this context, the main goal of artificial photosynthesis is to develop a photocatalytic system able to produce high energy content molecular fuels or feedstocks from water and/or CO2, as an approach to capture and storage solar energy.
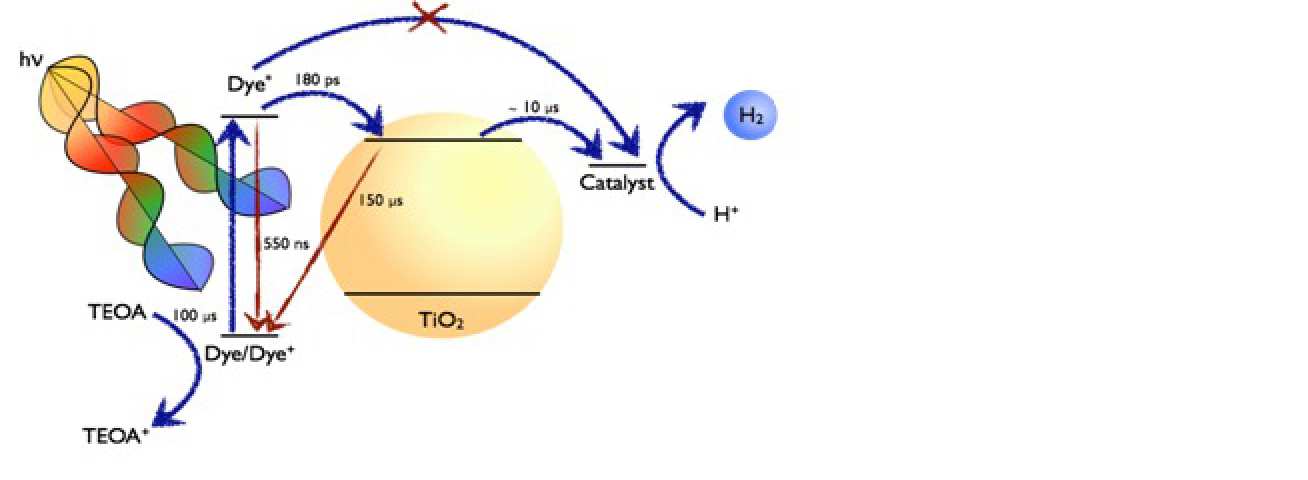
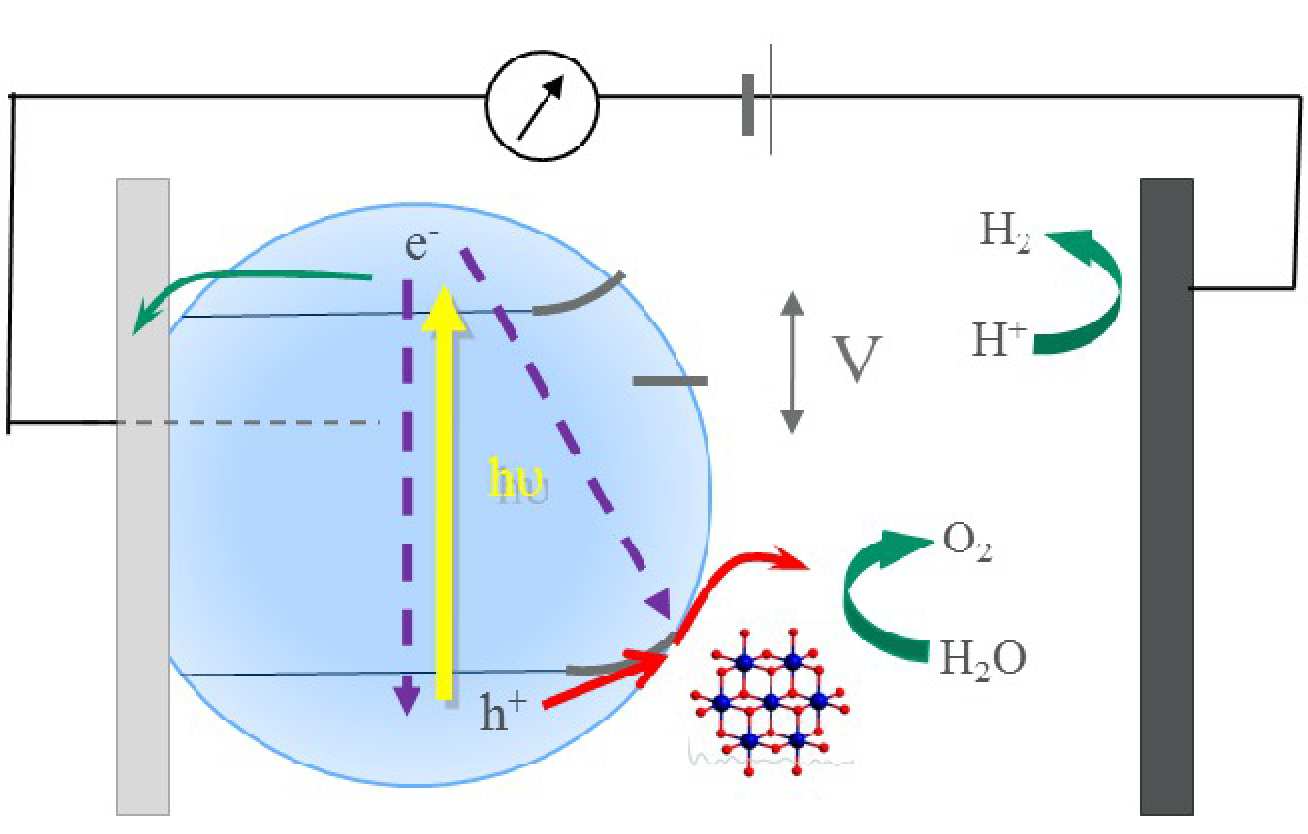
Our research addresses the photo(electro)chemical characterization and development of photoelectrodes and nanoparticles for solar driven fuel synthesis, with the primary focus being the light driven splitting of water into hydrogen and oxygen. In particular, we are interested in determining the kinetics and yields of charge separation and recombination, and the kinetics of water oxidation / reduction, using a suite of transient absorption spectrometers, complimented by impedance spectroscopies and photoelectrochemical measurements.
Our work on solar fuels involves collaborations across Imperial as part of the Imperial Artificial Leaf Initiative and across the UK as part of the UK Solar Fuels Network and externally, including in particular with EPFL (Prof Michael Gratzel), UCL (Prof Ivan Parkin and Dr Junwang Tang) and Cambridge University (Dr Erwin Reisner).
The photoactive materials employed in our studies primarily comprise metal oxide films and nanoparticles, with in some cases the addition of molecular dyes and catalysts. Two of our largest areas of study are:
(1) The study of nanostructured photoanodes able to oxidise water into oxygen
2H2O -> O2 + 4H+ + 4e-
Materials such as Fe2O3, BiVO4 or WO3 are potential candidates for solar water splitting photoelectrodes due to their narrow band gap (2.0 - 2.5 eV), which allow visible light harvesting of the solar spectrum, and a sufficiently oxidising valence band that allows the oxidation reaction of water into molecular oxygen. The work of our group is aimed at the determination of the lifetime of the photogenerated electron/hole pairs and the rates of charge carrier recombination and interfacial water oxidation. In order to enhance the photocatalytic activity of the water oxidation photoelectrodes, our group is studying the effects on charge carrier dynamics induced by material doping, incorporation of co-catalysts, changes in the morphology of the material and different surface treatments.
(2) The characterization of nanostructured and molecular catalyst-loaded nanostructured photocathodes able to reduce H+ or CO2 into fuels, such as H2, CO or methanol
2H+ + 2e- -> H2
CO2 + 6H+ + 6e- -> CH3OH
n-type and p-type semiconductors such as TiO2, ZrO2 or Cu2O, which have a conduction band above the reduction potential of H+ or CO2, can be used as photocathodes for the photochemical fuel synthesis. Our group is working on the synthesis and deposition of nanoparticulate semiconductors onto a solid support, in order to prepare and characterise mesoporous metal oxides photoelectrodes. Our research is mainly focused on the study of the kinetics of the electron transfer reactions that take place at the interface of the photoelectrodes and on the identification of the main charge carrier recombination reactions. In order to increase the lifetime of the charge carrier species, our work includes the preparation of inorganic heterojunctions, the coating of the semiconductior nanoparticles with protective blocking layers or the addition of co-catalysts to their surface. Our group is also investigating the effects on the selectivity and efficiency of the fuel production reaction when functionalizing the photoelectrodes with molecular or nanostructured catalysts.